Written by Craig Ford
The physical form of any personal care product is one of the first attributes that potential customers evaluate when considering purchasing a product, along with color and fragrance. If the form doesn’t allow for easy access from the package or smooth application, customers may not purchase or repurchase. Even the way a product flows while in the package can impact this perception. Another reason to measure this is to ensure that your products are uniform and reproducible. Although this is commonly referred to as viscosity, the accurate scientific term is rheology and there is more to it than simple viscosity.
IMPORTANT DEFINITIONS
Let’s define a few terms. According to Merriam-Webster:
Viscosity is the property of resistance to flow in any material with fluid properties.
and
Rheology is a science dealing with the deformation and flow of matter.
In other words, Rheology is the study of Viscosity under various conditions and therein lies the rub. The term viscosity is virtually useless without a clear understanding of the conditions under which it is measured. For many materials, temperature is the parameter which needs to be controlled and reported.
THE FORMULA FOR MEASURING VISCOSITY IS FAIRLY SIMPLE:
viscosity = shear stress / shear rate
Shear stress is the force per unit area required to move one layer of fluid in relation to another.
Shear rate is the measure of the change in speed at which intermediate layers move with respect to one another.
or
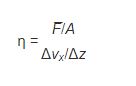
Where viscosity is represented by the symbol η “eta”. The more usual form of this relationship, called Newton’s equation, states that the resulting shear of a fluid is directly proportional to the force applied and inversely proportional to its viscosity. The similarity to Newton’s second law of motion (F = ma) should be apparent.
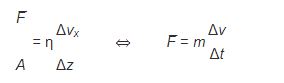
There are a few different units used in describing viscosity. Typical results are reported in centipoise (cP) which is equal to 1 millipascal second (mPa s) which also happens (coincidentally be almost exactly the viscosity of water at 20oC).
1 Pa s = 10 P
1,000 mPa s = 10 P
1 mPa s = 0.01 P
1 mPa s = 1 cP
There are a few different methods of measuring viscosity including the falling sphere method or capillary tube method but in the personal care industry, measuring the force required to rotate a spindle is the most commonly used method.
Newton assumed that viscosity and shear are always proportional and for many materials he was correct. These materials are said to exhibit Newtonian flow Characteristics.
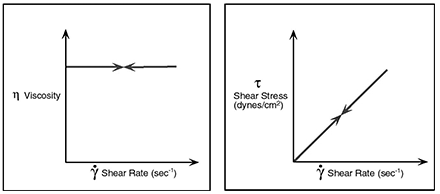
However, there are several types of non-Newtonian flow behavior, characterized by the way a fluid’s viscosity changes in response to variations in shear rate. The most common types of non-Newtonian fluids you may encounter include: Psuedoplastic, Dilatant and Plastic. Emulsions such as those is Cosmetics, are a common example of non-Newtonian pseudo-plastics.
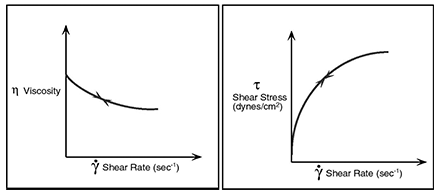
Rheology exists because these materials exhibit different flow characteristics with changing shear rates.
RHEOLOGY MODIFYING INGREDIENTS FOR COSMETICS:
There are a variety of ways of modifying the viscosity and rheology of cosmetic formulations primarily by choosing ingredients that inherently exhibit particular rheological properties. In addition, there are a variety of formulation additives available which act as rheology modifiers. A few classes of rheology modifiers are:
Mineral Colloidal Systems – Minerals (naturally sourced) such as Magnesium Aluminum Silicate, Bentonite, and Hectorite can be used to create colloidal systems which impart viscosity in a non-Newtonian manner. Typically synergistic when used with gums (xanthan). They also have a smooth, ‘dry’ feel. Hydrophobically modified minerals can also provide rheology modification in non-aqueous systems.
Polymeric Thickeners – These, usually acrylate based polymer thickeners, are cost effective and efficient at low use levels, can provide suspension of particles but can be sensitive to salt content and tend to short, choppy rheology.
Cellulosic Thickeners – Based on cellulose (wood pulp), these synthetically modified polymers are similar to the polymeric thickeners in that they can be highly efficient. At higher use levels they can feel slippery or stringy and do not provide suspending properties. Hydrophobic modification can allow for use in solvent systems.
Associative Thickeners – These thickeners interact with surfactants in a way that moderates flow and adds viscosity. They can be sensitive to different ingredients including sulfate free surfactants.
The information above is limited in scope and barely an introduction to this amazing area of science which is of critical importance to any cosmetic chemist. In addition to taking advantage of the SCC Continuing Education Program Courses, I recommend looking at the references below.
References