Written by Haley Gershon; Beta Analytic, www.betalabservices.com
Disclaimer: Beta is not affiliated with the BioPreferred program but is an approved laboratory.
APPLYING
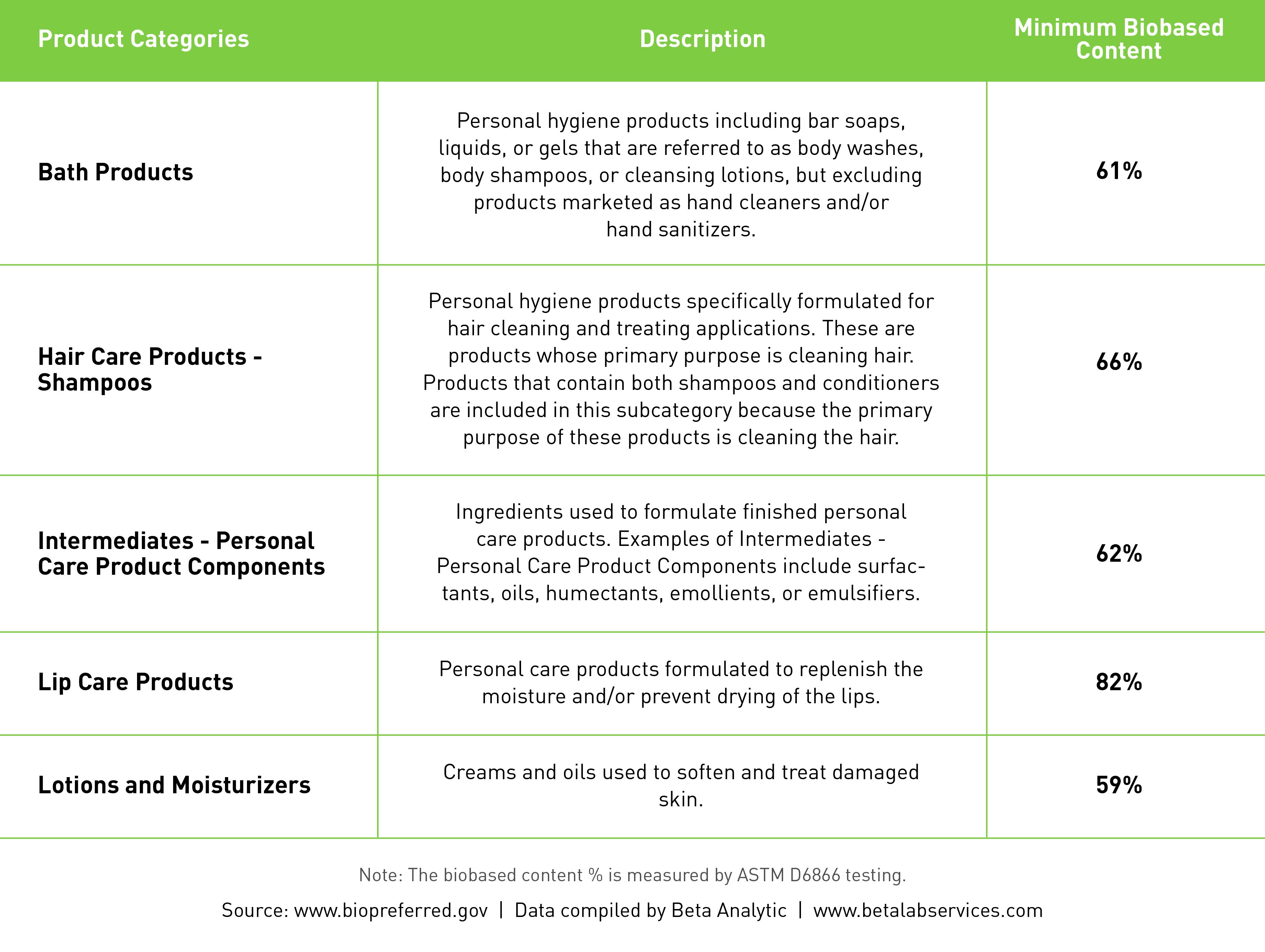
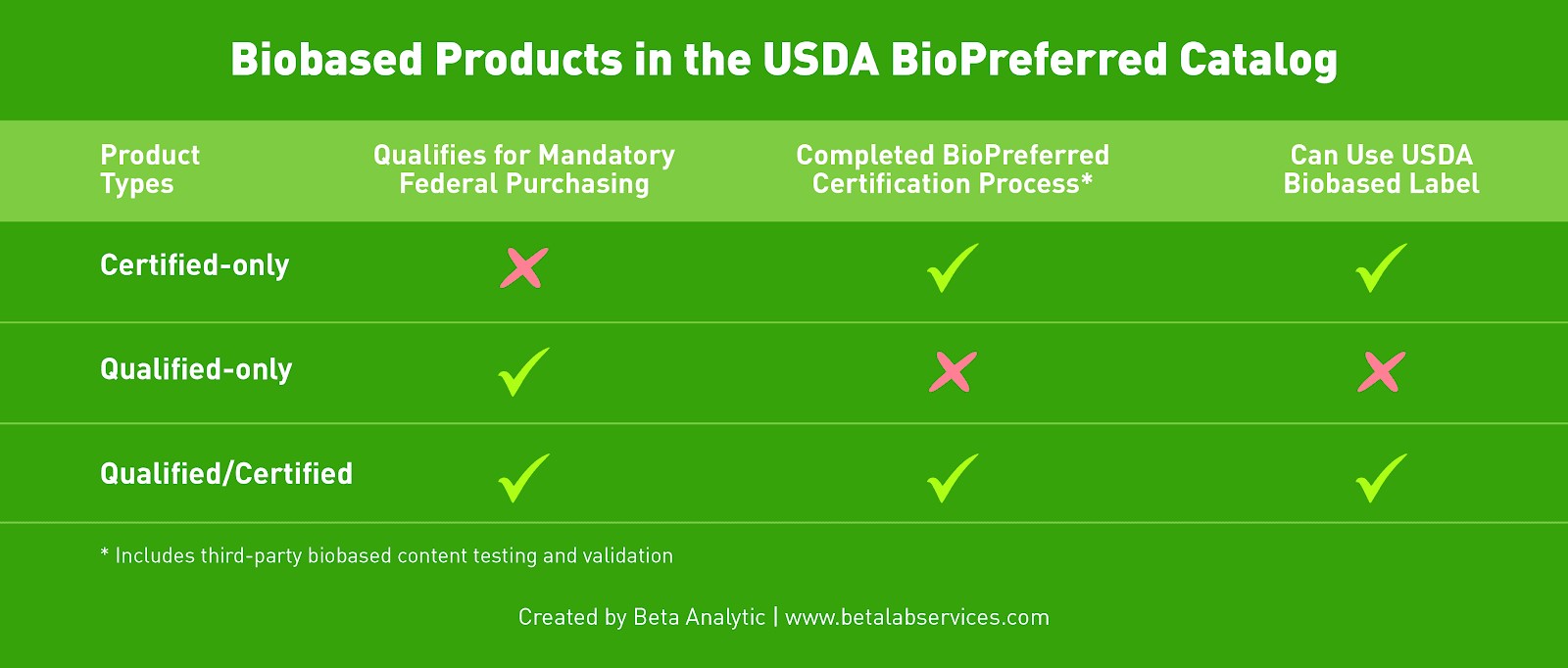
Manufacturers of cosmetic products containing biobased materials may apply for the USDA BioPreferred certification, allowing the use of the USDA Biobased label on eligible products. The program contains different product categories, each of which has a minimum biobased content required for certification as measured according to the ASTM D6866 test standard. For products which do not fall into an existing category, they must contain at least 25% biobased content to receive the BioPreferred certification.
The BioPreferred Voluntary Labeling program is open to all biobased products, including products imported for sale in the U.S. as long as they meet the biobased content requirements for the product category. Interested parties may also contact the USDA to get a preliminary assessment on product eligibility if the manufacturer is not based in the U.S.
The USDA BioPreferred certification is free so participants will only need to pay for the laboratory analysis of product samples.
APPLICATION PROCESS OVERVIEW
The first step of the certification process is to register for a Level 1 USDA eAuthentication Account in the USDA BioPreferred website. Once access is granted, businesses can register their company account within the Company Tools section of the BioPreferred database. Within the company account, participants can add products and apply for the certification. Applicants will then receive a notice confirming that the application is being processed.
Once the application is approved, participants will receive an Application ID and instructions to complete and send the Safety Equipment Institute (SEI) Biobased Participant Agreement to SEI. Once the application is confirmed, it is time for the product to undergo analysis.
Applications to the BioPreferred Program are evaluated on a first-come-first-serve basis.
ASTM D6866 BIOBASED TESTING BY AN APPROVED LABORATORY
To determine the biobased content of products with pending applications, an approved testing laboratory by the USDA BioPreferred program must complete the analysis. Approved laboratories are required to be ISO 17025-accredited, have no direct exposure to artificial carbon-14, and must demonstrate capability to perform ASTM D6866 testing.
Once an approved laboratory is contacted for analysis, participants have to provide the laboratory with their USDA Application Number and their SEI Documentation of Sample Selection, Shipping, and Disposal by Manufacturer/Vendor Form, which requires the following information:
- whether the sample is a retail or a non-retail product,
- the type of raw materials used to make the product,
- the number of tests to be conducted,
- if the sample contains inorganic carbonate,
- if the sample contains any hazardous or dangerous material, and
- any special care needed for sample disposal
Participants are required to include a copy of the form when sending a sample product to the testing laboratory. Prior to shipping samples, it is recommended to contact the laboratory for sample size requirements and turnaround time options.
Following receipt of the sample and completion of laboratory analysis, test results are sent to both the participant and SEI, who will review the result and submit it to the USDA. Test results are reported as percent biobased content to represent the portion of the material derived from biobased sources.
If the application is approved, the USDA will email the participant a Notice of Certification, allowing participants to display the BioPreferred Program label on their product packaging and marketing materials. If rejected, the participant will receive either a Notice of Denial or a Partial Certification email from the USDA. Rejected applicants may submit revised applications for reconsideration.
USDA BIOPREFERRED LABEL VALIDITY, AUDIT & RESTRICTIONS
The BioPreferred label can only be used on the biobased product identified in the application. Companies can only use the label upon receipt of the Notice of Certification from the USDA.
The certification remains valid only if the certified product complies with the required minimum biobased content. If the USDA changes the required minimum biobased content, the certified product must meet the new requirement to continue using the label.
To maintain the program’s credibility, the USDA routinely checks if the products are still eligible to use the label and that the contact and product information in the BioPreferred Catalog are updated. During the audit process, the USDA asks the program participants to verify that the formulation and manufacturing process of their products have not changed since certification.
Companies who failed to respond to the USDA were penalized by removing their certified products from the BioPreferred program database and online catalog.
For more information, visit www.biopreferred.gov.