WHY IS THIS IMPORTANT?
There are two crucial things to take from these descriptions and illustrations of fatty acid chains and their structural contribution to triglyceride composition and shape.
One, is that the relationship between the degrees of unsaturation of the fatty acid chains and the shape of the overall triglyceride molecule is what determines the melt point of the triglyceride (or rather the melt point of a significant mass of identically constituted triglyceride molecules). This relationship between unsaturation and molecular shape also relates to compatibility of different oils and fats, which we won’t discuss much here.
Two, is that the degrees of unsaturation within the fatty acids of a triglyceride determine the vulnerability of that triglyceride to degradation via oxidation (also to photodegradation).
So, a mass of oil or fat that has higher degrees of unsaturation within its constituent fatty acids will have a lower melt point and a greater vulnerability to oxidation. While a mass of oil or fat that has lower degrees of unsaturation within its constituent fatty acids will have a higher melt point and a lower vulnerability to oxidation.
Thus, degrees of unsaturation and melt point have an inverse relationship. That is, the more unsaturation, or the more double bonds present, the lower the melt point (Fig. 6). As well, the degrees of unsaturation and oxidative stability also have an inverse relationship. That is, the more unsaturation, or the more double bonds present, the lower the oxidative stability.
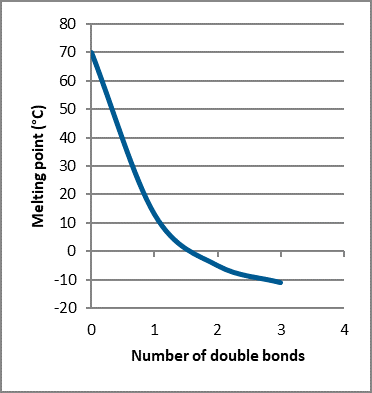
Fig. 6 – Melt point versus double bonds (unsaturation) present in a fatty acid chain
(One thing that we’re not considering for the sake of simplicity is the chain length of the fatty acids, though this can be a significant factor in melt point, and to a lesser extent oxidative stability. Though, it should be noted that the degree of unsaturation has a more significant effect on melt point than chain length for the fatty acid range that we typically consider in plant oils and fats. An example of this would be that the melt point difference between Lauric Acid C12:0 and Stearic Acid C18:0 is about 30C degrees, while the difference in melt point between Stearic Acid C18:0 and Oleic Acid C18:1 is about 60C degrees.)
WHAT’S NEXT?
There is a deeper conversation to be had about how individual triglyceride molecules line up when crystallized into a solid, and how this ability of the molecules to stack and align in a particular fashion is really the mechanism that determines the melt point of individual fats oils and the compatibility between different oils and fats, but that’s beyond the scope of this post.
As well, there is another conversation to be had concerning how the double bonds present in mono-unsaturated and poly-unsaturated fatty acids are the sites of vulnerability to attack by oxygen atoms, and how that process occurs chemically, but again that is beyond the scope of this article.
(One last technical point is that the analytic measurement used to determine the degree of unsaturation is know as Iodine Value. If you ever see Iodine Value, or IV, on a specification sheet this refers directly to a measurement of how many double bonds there are in a mass of oil or fat. The units are essentially arbitrary, but the typical range is about 0-200, with most natural oils falling in between these two limits. For example, Coconut Oil has an IV of about 8-12, while Flaxseed Oil is about 178.)
Now that we have a decent understanding of fatty acid saturation and how it determines the characteristics of a triglyceride, let’s look at a couple of examples of actual oils and fats.
OILS AND FATS
The first is Coconut Oil, which is a fairly simple example, as it is composed almost entirely of saturated fatty acids (Fig. 7).
Coconut Oil Fatty Acid Composition
Fatty Acid Chain Length ---- Percentage
Caprylic Acid C8:0 ---- 9
Capric Acid C10:0 ---- 6
Lauric Acid C12:0 ---- 47
Myristic Acid C14:0 ---- 18
Palmitic Acid C16:0 ---- 9
Stearic Acid C18:0 ---- 3
Oleic Acid C18:1 ---- 6
Fig. 7 – Coconut Oil Fatty Acid Composition. Dominated by Lauric Acid, a medium chain, saturated fatty acid.
We can see that only about 6% of the triglycerides of Coconut Oil are composed of unsaturated fatty acids. Thus, given what we just reviewed, we should expect that Coconut Oil will have a relatively higher melt point and that it should be relatively stable against oxidation. And that is what we see. Anyone who has seen a jar of Coconut Oil knows that it is solid or semi-solid at room temperature (~22°C), as opposed to most common oils which are fully liquid at room temperature. And anyone who has kept a jar of Coconut Oil in their pantry, or lab, for a significant amount of time will know that it rarely goes rancid. And now we know that these two characteristics of Coconut Oil, solidity at room temperature and long-term stability, are directly determined by its fatty acid composition.
Let’s look at one more example to close out our discussion.
Corn oil is another common oil but it has a fatty acid composition that is markedly different from Coconut Oil (Fig. 8).
Corn Oil Fatty Acid Composition
Fatty Acid Chain Length ---- Percentage
Palmitic Acid C16:0 ---- 10
Stearic Acid C18:0 ---- 2
Oleic Acid C18:1 ---- 29
Linoleic Acid 18:2 ---- 56
Linolenic Acid 18:3 ---- 3
Fig. 8 – Corn Oil Fatty Acid Composition. Dominated by Linoleic Acid, a long chain, polyunsaturated fatty acid.
When we look at the composition of Corn Oil, we see that it is composed of only about 12% saturated fatty acids, and almost 60% polyunsaturated fatty acids. Thus, we should expect that it will have a relatively low melt point and should be relatively unstable against oxidation. And again, that is what we see. Of course, Corn Oil is liquid at room temperature, so its melt point is somewhat irrelevant for our purposes. However, we can instead talk about its freeze point, which is about -11°C. This is well below the freezing point of water. So, while Coconut Oil is typically solid at room temperature, Corn Oil will remain liquid well past the point of ice forming. That’s a difference of about 35C degrees. Now we can really start to see how the fatty acid composition of different oils can affect their respective melting and freezing points.
Additionally, we can look at the oxidative stability of these two example oils. Corn Oil has a relatively low oxidative stability because it’s high poly-unsaturated fatty acid content drastically increases its vulnerability to degradation relative to an oil like Coconut Oil. And if you’ve ever looked at the specification sheet of Corn Oil that is intended for use in a personal care or cosmetic product, you will likely see that it has some form of antioxidant added. This is necessary for the oil to maintain a decent shelf life. Whereas Coconut Oil will rarely, if ever, have an anti-oxidant added. (In the past, this would have been synthetic compounds like TBHQ, BHT, or BHA. However, as the market calls for more natural solutions we have seen increased use of Tocopherols and Rosemary Extract.)
The last point I’ll make about these two oils is that they are not very compatible. That is, if you were to use them at equivalent levels in the oil phase of an emulsion, or an anhydrous product, they would not want to form a very stable, homogenous mixture. And this is again because of the differences in their fatty acid compositions. Their differences of medium chain versus long chain, saturated versus poly-unsaturated, and higher versus lower melt points do not allow for their triglyceride molecules to line up well with each other. They’re different shapes and because of this they want to crystallize at drastically different temperatures. Because of this incompatibility we would want to choose one oil as the major portion and the other as a minor portion. Such that there is at least a 2:1 ratio.
CONCLUSION
Hopefully, this brief comparison of two common oils has given us some perspective as to how differences in triglyceride and fatty acid composition directly determine the unique characteristics of different oils and fats.
We could spend a lot more time getting into the details of what we’ve discussed here, as well as further comparisons of fats like Shea Butter or oils like High Oleic Sunflower Oil, but that will have to be for another time.